The Ryan Haight Act Known as
Online Pharmacy Consumer Protection Act of 2008
Sec. 2. Requirement of a valid prescription for
controlled substances dispensed by means of the Internet.
SUMMARY: The Ryan Haight Online Pharmacy Consumer Protection Act,
which was enacted on October 15, 2008,amended the Controlled Substances Act and Controlled Substances Import and Export Act by adding several new provisions to prevent the illegal distribution and dispensing of controlled substances by means of the Internet.

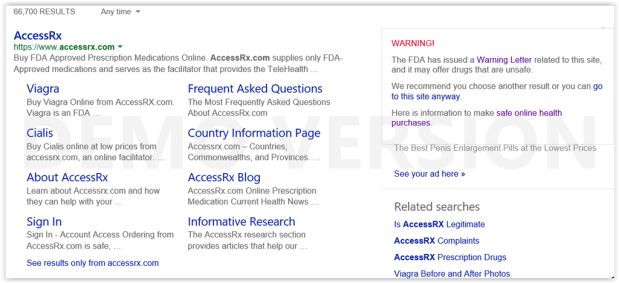
Legitscript Research On Internet Pharmacy Affiliate Networks
Who is Secure Medical?
Secure Medical, Inc. (SMI) is a medical technology company that was established in 1998. The birth of the internet gave SMI a great opportunity to improve the delivery of healthcare from Clinics and Pharmacies to effectively increase the awareness of products and services offered to their Patients. SMI has a primary objective to become a central creative group to develop, manage and support the technical process which drives remote healthcare. SMI found the opportunity to promote Telemedicine which has proven to be an integral part of the growth and expansion of the company. Advancement in healthcare technology with patient care was destined to improve and SMI decided early on that they would support their proprietary services by partnering with key Corporations, State Government, Associations and Affiliations
In lay man terms, Secure Medical is a internet marketing company……………..
AccessRx Contact Information
Mailing Address:
ACCESSRX.COM c/o Secure Medical
1155 W. Rio Salado Pkwy
Suite 201
Tempe, AZ 85281 USA
Phone Number:
1-800-467-0297 [Toll-free USA Only]
Monday – Thursday, 6am to 8pm, • Friday, 6am to 7pm
Saturday, 7am to 5pm, MST
Sun. CLOSED
E-mail Address:
arxservice@accessrx.com
Billing Provided By:
Secure Medical
1155 W. Rio Salado Pkwy
Suite 201
Tempe, AZ 85281 USA
Ph# 480-505-5700
| AccessRx | http://www.accessrx.com |
| AccessRx | http://www.bigmeds.com |
| AccessRx | http://www.privatepills.com |
| AccessRx | http://www.coloradomeds.com |
| AccessRx | http://www.radiomeds.com |
| AccessRx | http://www.consumermeds.com |
| AccessRx | http://www.peoplemeds.com |
| AccessRx | http://www.tvmeds.com |
| AccessRx | http://www.medstoday.com |
| AccessRx | http://easyemeds.com |
| AccessRx | http://easyemeds.net |
| AccessRx | http://hugemeds.com |
| AccessRx | http://totalmeds.com |
| AccessRx | http://totalpills.com |
| AccessRx | http://localmeds.com |
| AccessRx | http://medmachine.com |
| Access RX | http://www.access-rx.com |
- AccessRx http://www.bigmeds.com
- AccessRx http://www.privatepills.com
- AccessRx http://www.coloradomeds.com
- AccessRx http://www.radiomeds.com
- AccessRx http://www.consumermeds.com
- AccessRx http://www.peoplemeds.com
- AccessRx http://www.tvmeds.com
- AccessRx http://www.medstoday.com
- AccessRx http://www.accessrx.com
- AccessRx http://easyemeds.com
- AccessRx http://easyemeds.net
- AccessRx http://hugemeds.com
- AccessRx http://localmeds.com
- AccessRx http://medmachine.com
- AccessRx http://totalmeds.com
- AccessRx http://totalpills.com
- stimula.us
- viamedic.us
- lcpharmacy.org
- pharmacyreview.org
- accessrx.net
- easyemeds.net
- lcpharmacy.net
- lowcostpharmacy.net
- pharmacyreview.net
- via100.net
- via1000.net
- viapro.net
- oaktreedrugs.net
- edrugstore-md.net
- openmindsproductions.net
- openmindstv.net
- openmindsnetwork.net
- openmindsproduction.net
- openmindstelevision.net
- pillmaster.net
- compareviagraprices.net
- discountpills.net
- expresspills.info
- accessrx.com
- all-viagra.com
- amerimedrx.com
- aperx.com
- apexrx.com
- devsm.com
- easyemeds.com
- edrugsonline.com
- empiredrugs.com
- fastpharmacy.com
- globalmedications.com
- healthymale.com
- lcpharmacy.com
- lcppharmacy.com
- levitra-us.com
- lowcostpharmacy.com
- medfico.com
- Domain
- pharmacyreview.com
- pharmgroup.com
- pilldr.com
- pilldrugstore.com
- pillrx.com
- pillusa.com
- pilusa.com
- premiumdrugs.com
- promedicrx.com
- propecia-us.com
- rbhealth.com
- rootspharmacy.com
- rxbuyingclub.com
- safeprescribe.com
- sechosting.com
- secure-medical.com
- securemedical-affiliate.com
- securemedical.com
- securemedicalaffiliates.com
- securepharmacy.com
- smclients.com
- stimula-us.com
- ultimaterx.com
- us-cialis.com
- via100.com
- via1000.com
- viagrabargain.com
- viamedic.com
- viamedicinternational.com
- viapro.com
- vimedic.com
- consumermeds.com
- privatepills.com
- medstoday.com
- hugemeds.com
- expresspills.com
- localmeds.com
- healthkiosk.com
- totalpills.com
- totalmeds.com
- coloradomeds.com
- healthkiosks.com
- tvmeds.com
- radiomeds.com
- bigmeds.com
- peoplemeds.com
- edrugstore-md.com
- medmachine.com
- smstaging.com
- premieretelemedicine.com
- chatsm.com
- oaktreedrugs.com
- openmindsproduction.com
- openmindstelevision.com
- openmindstv.com
- awmerirx.com
- openmindsmagazine.com
- pilldoc.comAccessRx http://www.blue-pill.com

Legitscript Research On Internet Pharmacy Affiliate Networks
TO: Secure Medical Inc.
Philip Hamilton
1155 W. Rio Salado Parkway, Suite 201
Tempe, AZ 85281
sysadmin@securemedical.com
FROM: United States Food and Drug Administration
Center for Drug Evaluation and Research
Office of Compliance
Office of Drug Security, Integrity and Recalls
Division of Supply Chain Integrity
RE: Internet Marketing of Unapproved and Misbranded Drugs
DATE: February 11, 2013
WARNING LETTER
The United States Food and Drug Administration (FDA) recently reviewed your websites “http://www.viamedic.com”, “http://www.accessrx.com”, and “http://www.AmeriMedixRx.com” and has determined that your websites offer products for sale in violation of the Federal Food, Drug, and Cosmetic Act (FD&C Act). More specifically, your websites offer unapproved and misbranded new drugs for sale in violation of sections 502(a), 502(f)(1), 503(b)(1), and 505(a) of the FD&C Act [21 U.S.C. §§ 352(a), 352(f), 353(b), and 355(a)]. Introduction of such products into interstate commerce is prohibited under sections 301(a) and 301(d) of the Act [21 U.S.C. §§ 331(a) and 331(d)]. We request that you immediately cease marketing violative drug products to United States consumers.
Unapproved New Drugs
Your firm offers for sale through your websites “Tamiflu 75mg Gel Tablet.” Tamiflu is a proprietary name of an FDA-approved drug, oseltamivir phosphate, well known for its intended use to treat disease (influenza). FDA has not, however, approved any Tamiflu gel tablet products.
The oseltamivir phosphate you offer for sale through your websites is a drug within the meaning of section 201(g) of the FD&C Act [21 U.S.C. § 321(g)] because it is intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease and/or it is intended to affect the structure or function of the body. This product is also a new drug as defined by section 201(p) of the FD&C Act [21 U.S.C. § 321(p)], because it is not generally recognized as safe and effective for the labeled uses. No approved applications pursuant to section 505 of the FD&C Act [21 U.S.C. § 355] are in effect for a Tamiflu gel tablet product. Accordingly, their introduction or delivery for introduction into interstate commerce violates section 505(a) of the FD&C Act [21 U.S.C. § 355(a)], which is prohibited under section 301(d) of the FD&C Act [21 U.S.C. § 331(d)].
Misbranded Drugs
These drugs are misbranded in several different ways: Your websites offer prescription drugs for sale without requiring that the drugs be dispensed only upon a prescription from a practitioner licensed by law to administer such drugs. Therefore, the drugs are misbranded under section 503(b)(1) of the FD&C Act [21 U.S.C. § 353(b)(1)]. If Tamiflu or its active ingredient (oseltamivir phosphate) is not taken under close supervision of a healthcare professional and pharmacist, it can be a potentially dangerous prescription drug.
These drugs are misbranded pursuant to section 502(a) of the FD&C Act [21 U.S.C. 352(a)] because their labeling is false or misleading. Your firm’s websites and promotional labeling for this product misleads the consumer to believe that the “Tamiflu 75mg Gel Tablet” they are purchasing is an FDA approved drug product.
Additionally, because the above mentioned drug is intended for the treatment of conditions that are not amenable to self-diagnosis and treatment by individuals who are not medical practitioners, adequate directions cannot be written for them so that a layman can use these products safely for the intended uses. Consequently, the labeling fails to bear adequate directions for the intended uses, causing the drug to be misbranded under 502(f)(1) of the FD&C Act [21 U.S.C. § 352(f)(1)]. Because “Tamiflu 75mg Gel Tablet” offered for sale on your websites lack a required approved application, it is not exempt from the requirements of section 502(f)(1) of the FD&C Act [21 U.S.C. § 352(f)(1)] as described in Title 21 of the Code of Federal Regulations (21 CFR) § 201.115. The introduction or delivery for introduction into interstate commerce of a misbranded drug product is a prohibited act under section 301(a) of the FD&C Act [21 U.S.C. §331(a)].
FDA is taking this action against your firm because of the inherent risk in buying unapproved and misbranded new drugs. Unapproved new drugs from unregulated sources do not have the same assurance of safety and effectiveness as those drugs subject to FDA oversight, and such drugs have been found to be contaminated, counterfeit, contain varying amounts of active ingredients, or contain different ingredients altogether.
This letter is not intended to identify all the ways in which your activities might be in violation of law. It is your responsibility to ensure that all products marketed by your firm are in compliance with the FD&C Act and its implementing regulations. You should take prompt action to correct the violations noted above. Failure to correct these violations promptly may result in regulatory action, including but not limited to seizure and/or injunction without further notice.
Please notify this office within 15 working days of receipt of this letter of any steps you have taken or will take to correct the noted violations and to prevent their recurrence. If the corrective action(s) cannot be completed within 15 days, state the reason for the delay and the time within which the correction(s) will be completed. Your response and any other inquiries concerning this letter should be sent to FDA’s Internet Pharmacy Task Force at FDAInternetPharmacyTaskForce-CDER@fda.hhs.gov.
Sincerely,
/S/
Howard Sklamberg, Director
Office of Compliance
Center for Drug Evaluation and Research
cc: John Rao
5508 S. Waverly Way
Tempe, AZ 85283-2024
Aug 19 – Dec 18 5% Off All ED Medication AccessRx
Aug 19 – Dec 18 Chantix 0.5 (11)-1 at just $9 … AccessRx
Aug 19 – Dec 18 Nasonex 50Mcg at $329 … AccessRx
Can I order viagra from the chemist
Trident Media (blog)-Sep 27, 2017
You can buy Viagra online without prescription in hand at AccessRx! Brand and generic Viagra for sale. Online pharmacy forum What New Buy …
Money and Prescribing: A Case Study in One American City
Medscape-Aug 14, 2017
An analysis of data reported to the AccessRx program in Washington, DC, which tracks pharmaceutical marketing expenses, found that …